Primary Batteries :
`color{green}text(Definition) :` In the primary batteries, the reaction occurs only once and after use over a period of time, battery becomes dead and cannot be reused again.
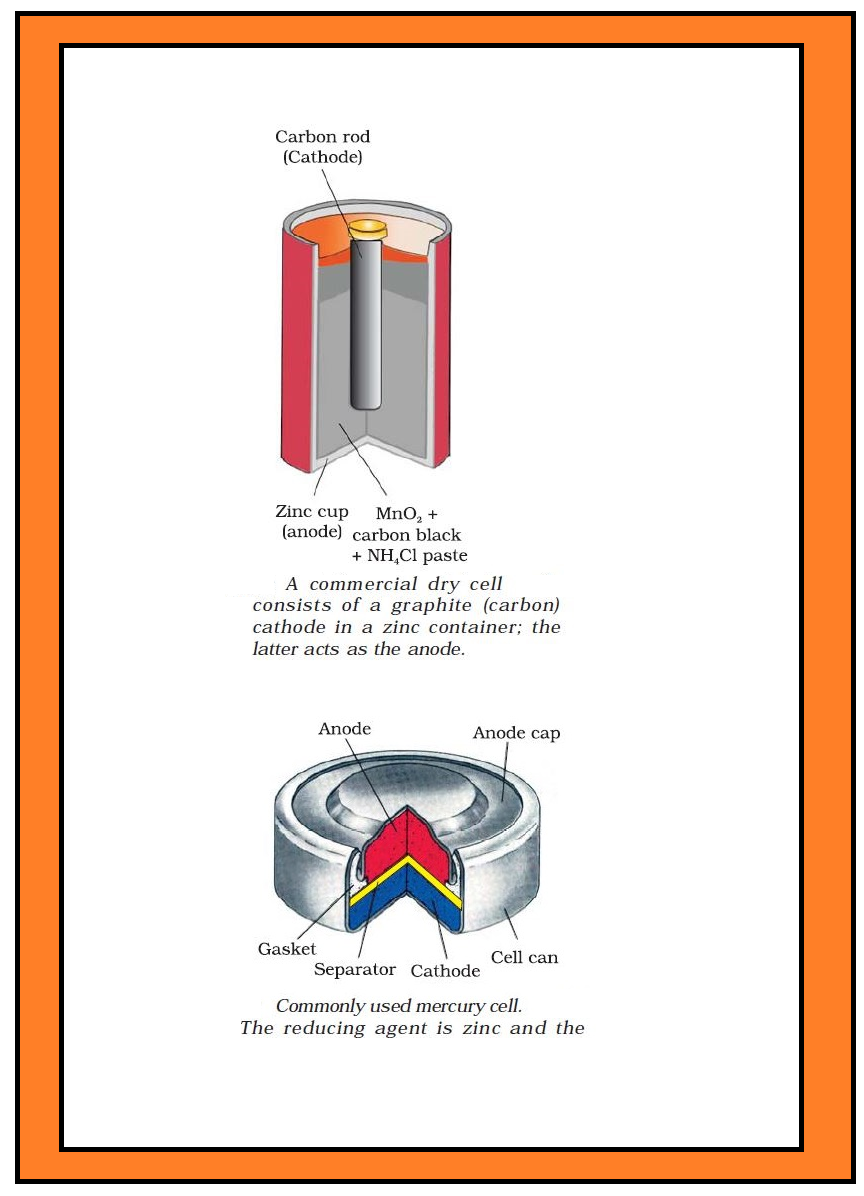
`color{green}text(Examples) :` (i) Dry cell (known as Leclanche cell after its discoverer) which is used commonly in our transistors and clocks.
`color{green}text(Composition and Working) :`
`=>` `color{green}("Anode")` : a zinc container.
`=>` `color{green}("Cathode")` : A carbon (graphite) rod surrounded by powdered manganese dioxide and carbon (Fig.3.8).
`=>` The space between the electrodes is filled by a moist paste of ammonium chloride `(NH_4Cl)` and zinc chloride `(ZnCl_2)`.
`=>` The electrode reactions are complex, but they can be written approximately as follows :
`color{green}("Anode")` : `color{red}(Zn (s) → Zn^(2+) + 2 e^(-))`
`color{green}("Cathode")` : `color{red}(MnO_2+NH_4^(+) + e^(-) → Mn O ( OH) + NH_3)`
`=>` In the reaction at cathode, manganese is reduced from the `+ 4` oxidation state to the `+3` state.
`=>` Ammonia produced in the reaction forms a complex with `Zn^(2+)` to give `[Zn (NH_3)_4]^(2+)`.
`=>` The cell has a potential of nearly `1.5 V`.
(ii) Mercury cell, (Fig. 3.9) suitable for low current devices like hearing aids, watches, etc.
`color{green}text(Composition and Working) :`
`=>` `color{green}("Anode")` : Zinc–mercury amalgam.
`=>` `color{green}("Cathode")` : A paste of `HgO` and carbon.
`=>` `color{green}("Electrolyte")` : Paste of `KOH` and `ZnO`.
`=>` The electrode reactions for the cell are given below :
`color{green}("Anode")` : `color{red}(Zn (Hg) +2 OH^(-) → ZnO(s) +H_2O + 2 e^(-))`
`color{green}("Cathode")` : `color{red}(HgO +H_2O +2 e^(-) → Hg (l) +2OH^(-))`
The overall reaction is represented by :
`color{red}(Zn (Hg) +HgO (s) → ZnO(s) +Hg(l))`
`=>` The cell potential is approximately `1.35 V` and remains constant during its life as the overall reaction does not involve any ion in solution whose concentration can change during its life time.
`color{green}text(Examples) :` (i) Dry cell (known as Leclanche cell after its discoverer) which is used commonly in our transistors and clocks.
`color{green}text(Composition and Working) :`
`=>` `color{green}("Anode")` : a zinc container.
`=>` `color{green}("Cathode")` : A carbon (graphite) rod surrounded by powdered manganese dioxide and carbon (Fig.3.8).
`=>` The space between the electrodes is filled by a moist paste of ammonium chloride `(NH_4Cl)` and zinc chloride `(ZnCl_2)`.
`=>` The electrode reactions are complex, but they can be written approximately as follows :
`color{green}("Anode")` : `color{red}(Zn (s) → Zn^(2+) + 2 e^(-))`
`color{green}("Cathode")` : `color{red}(MnO_2+NH_4^(+) + e^(-) → Mn O ( OH) + NH_3)`
`=>` In the reaction at cathode, manganese is reduced from the `+ 4` oxidation state to the `+3` state.
`=>` Ammonia produced in the reaction forms a complex with `Zn^(2+)` to give `[Zn (NH_3)_4]^(2+)`.
`=>` The cell has a potential of nearly `1.5 V`.
(ii) Mercury cell, (Fig. 3.9) suitable for low current devices like hearing aids, watches, etc.
`color{green}text(Composition and Working) :`
`=>` `color{green}("Anode")` : Zinc–mercury amalgam.
`=>` `color{green}("Cathode")` : A paste of `HgO` and carbon.
`=>` `color{green}("Electrolyte")` : Paste of `KOH` and `ZnO`.
`=>` The electrode reactions for the cell are given below :
`color{green}("Anode")` : `color{red}(Zn (Hg) +2 OH^(-) → ZnO(s) +H_2O + 2 e^(-))`
`color{green}("Cathode")` : `color{red}(HgO +H_2O +2 e^(-) → Hg (l) +2OH^(-))`
The overall reaction is represented by :
`color{red}(Zn (Hg) +HgO (s) → ZnO(s) +Hg(l))`
`=>` The cell potential is approximately `1.35 V` and remains constant during its life as the overall reaction does not involve any ion in solution whose concentration can change during its life time.